


Nitrogen
(N)
Nitrogen is generally the first factor limiting plant growth, with the exception of legumes, which are the only botanical family capable of directly absorbing the nitrogen from the air through symbiosis with bacteria present in the form of nodules on their roots.
Nitrogen is a nutrient that is indispensable to plant growth, as it allows plants to build up proteins, chlorophyll, enzymes and vitamins. It is therefore the main factor for plant growth, and also determines quality.
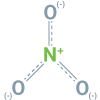
Ammonium, nitrate, and urea are the three forms of nitrogen (N) contained in fertilizers. While nitrate (NO3-) and ammonium (NH4+) are immediately available for the crops after application (1), urea needs to be converted (hydrolysis, 7) to NH4+.
Nitrate is the preferred N-form, as it is water-soluble and therefore immediately plant available (2). It enhances the uptake of cations, such as K+, Ca2+, Mg2+. Part of the ammonium can also be directly absorbed by the crops (uptake, 3) and depending on soil characteristics, NH4+ is also converted into NO3- (nitrification, 4).
Denitrification (5) is a process where NO3- is reduced to nitrite (NO2-), nitric oxide (NO), nitrous oxide (N2O) and N2. This reaction is mediated by anaerobic bacteria, and therefore occurs in anoxic environments and is thus scarce in well-aerated agricultural soils. As an ion, NO3- is also quite mobile in the soil and can be leached with excess rainfall (leaching, 9). Therefore, it is important to split high amounts of N fertilization into several smaller application rates and to fertilize at the right time, when the demand of the crop is high.
The soil microorganisms consume mainly NH4+ but also NO3- (immobilization, 6a). The presence of carbon-rich but nitrogen-poor organic matter (for instance straw) enhances immobilization. However, this proportion of N is not lost and becomes plant available later when biomass including the microbial biomass decomposes (mineralization, 6b).
After soil application, urea ((NH2)2CO) breaks down to two molecules of ammonia (NH3) and one molecule of carbon dioxide (CO2). The gaseous NH3 can escape into the atmosphere (volatilization, 8). The reaction of NH3 with water (H20) to form NH4 releases a hydroxide ion (OH-) and thus increases the soil pH. Ammonia volatilization is particularly high in alkaline soils (pH>7). Therefore, temporary pH increases in soil pH facilitates high volatilization losses even on acidic soils.
Very
Fairly
Moderately
| N | ||
|---|---|---|
| Cabbage | ||
| Carrot | ||
| Lettuce | ||
| Grain Maize | ||
| Silage Maize | ||
| Tomato | ||
| Winter Rapeseed | ||
| Winter Wheat | ||
| Starch Potato | ||
| Cucumber | ||
| Sugar Beet | ||
| Spring Barley | ||
| Strawberry | ||
| Winter Barley | ||
| Fiber Flax | ||
| Sunflower | ||
| Apple | ||
| Pear | ||
| Cherries | ||
| Grape Vine |
Sensitivity table
Nitrogen is a nutrient that is indispensable to plant growth, as it allows plants to build up proteins, chlorophyll, enzymes and vitamins. It is therefore the main factor for plant growth, and also determines quality.
When nitrogen nutrition is disturbed, the different parts of the plant are smaller, and yields are reduced.
For grains, nitrogen is decisive for obtaining a high protein rate: after the variety, it is the main lever for increasing the protein content. All varieties of soft wheat are adversely affected by nitrogen deficiency. Losses depend on the intensity of the deficiency and its duration (total time of the deficiency and periods of the cycle affected). Early deficiencies at the start of stem elongation are the most harmful for yield, as they occur at a time when the need for nitrogen is highest.
Symptoms
Insufficient nitrogen nutrition leads to reduced protein synthesis, which has a damaging effect on plant growth and development.
Nitrogen-deficient plants show yellowing due to inadequate chlorophyll synthesis and the drying of older leaves.
Excess
Excessive nitrogen fertilization is not desirable, neither from a farming (risk of lodging), cost (waste) or environmental (risk of leaching) point of view. In order to avoid loss and fertilize efficiently, LAT Nitrogen recommends the use of N-Pilot®.
That is why many tools have been developed to identify the dose to apply for achieving optimum yield.
Requirements
The nitrogen requirements of the plant depend on the species, variety and expected yield. Nitrogen fertilization can be calculated precisely depending on the needs of the crop and the quantity supplied by the soil.
Although ammonia synthesis was first demonstrated in 1909, it was only after the Second World War that it gained importance for agriculture. Today ammonia, which is the starting molecule for all nitrogen fertilizers, is synthesized using the Haber-Bosch process. This process had an enormous impact on mankind and is often hailed as the most important invention of the 20th century. Both inventors Fritz Haber and Carl Bosch received the Nobel prize.
The process converts atmospheric nitrogen into ammonia through a reaction with hydrogen. The main hydrogen source is methane (CH4) from natural gas. It is therefore an energy intensive process.
Ammonium nitrate is produced by an acid-base reaction of ammonia with nitric acid. The result is a white crystal solid, which is highly soluble in water. Calcium ammonium nitrate is the product when calcium nitrate reacts with ammonia. Urea is produced from ammonia and carbon dioxide and UAN is a mixture of ammonium nitrate and urea. The combined solubility is much higher than that of each component alone. UAN is therefore used in liquid solution.
The measurement of mineral nitrogen at the end of winter by analysing the soil makes it possible to assess the availability of the element to the plant before vegetation restarts in early spring, which is a period of intense uptake. During the season, decision-making tools such as the N-Pilot® help to adjust the nitrogen dose.
LAT Nitrogen Austria GmbH
St.-Peter-Strasse 25
4021 Linz, Austria